

 |
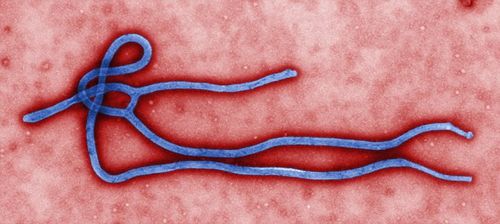
| Ebola virus. (File photo) |
The restructured Ebola vaccine independently developed by China entered phase two clinical trials in Sierra Leone on Oct. 11, 2015. This is also the first time that this Chinese Ebola vaccine has received an overseas clinical trial permit, Xinhua reported.
The Chinese Ebola vaccine has three major features. Firstly, the vaccine is the first based on the Ebola virus strain responsible for the West African outbreak in 2014. Until now, all tested Ebola vaccines were based on a strain from the Zaire outbreak of 1976. Secondly, the vaccine is currently in the form of a freeze-dried powder, which will keep it stable for at least two weeks in temperatures of up to 37 degrees Celsius. This will make it suitable for the tropical West African region and assist with large-scale production; and thirdly, clinical outcomes have proved the safety and efficacy of the vaccine.
Phase one of clinical trials of the Chinese Ebola vaccine that included human testing indicates that the vaccine is safe.
 Winding mountain road
Winding mountain road
 Math teacher makes 'solar powered electric car'
Math teacher makes 'solar powered electric car' Heavy traffic turns expressway into huge parking lot
Heavy traffic turns expressway into huge parking lot Chinese-American girl selected as Rose Parade princess
Chinese-American girl selected as Rose Parade princess Top 10 nominated designs at BJDW
Top 10 nominated designs at BJDW Fashion show staged in Forbidden City at night
Fashion show staged in Forbidden City at night Construction of HK-Zhuhai-Macao Bridge enters final stage
Construction of HK-Zhuhai-Macao Bridge enters final stage Model of heavy-lift copter makes debuts at Tianjin expo
Model of heavy-lift copter makes debuts at Tianjin expo Art photos of Chinese beauty in Han Chinese clothing
Art photos of Chinese beauty in Han Chinese clothing Silent march
Silent march Assassins for love
Assassins for love Ankara bombs stress danger of extremism
Ankara bombs stress danger of extremism Stock volatility has little impact on China's economy
Stock volatility has little impact on China's economyDay|Week